CLINICAL RESEARCH
CLINICAL OPERATIONS
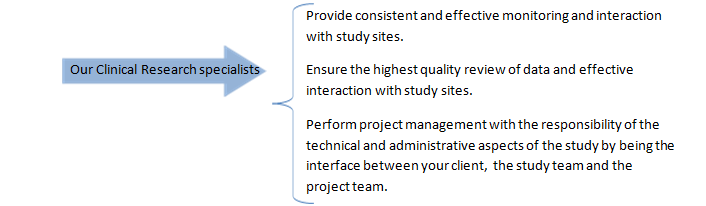
We conduct on-site monitoring visits throughout the study to:
- Undertake site management
- Oversee data collection, manage monitoring plans, timing and logistics
- Review source documentation and Case Report Forms
- Manage Adverse Event reporting
- Perform Source Data Validation
- Therapeutic units / study devices management
- Ensure Regulatory Compliance
- Resolve data queries
And also:
- Conduct investigator pre-study meeting
- Training of site study team
- Assist the co-ordination of investigator meeting
- Conduct study initiation site visits
- Undertake study closure visits
We provide support:
- On investigators on site
- In pre-screening of patients
- In eCRF filling
With project management experiences:
- We serve as a primary resource and point of communication for the client and project team
- We provide leadership and direction to the project team, including specifications for time, quality and cost of deliverables
- We use capabilities, systems and processes to assure efficiency and accuracy throughout the project
- We create a management framework to optimize the skills of functional leads, and drives focus on the quality and efficiency of individual tasks
- We follow global risk management process, addressing potential obstacles to project delivery
- We maintain a flexible, can-do approach to address issues innovatively and proactively








